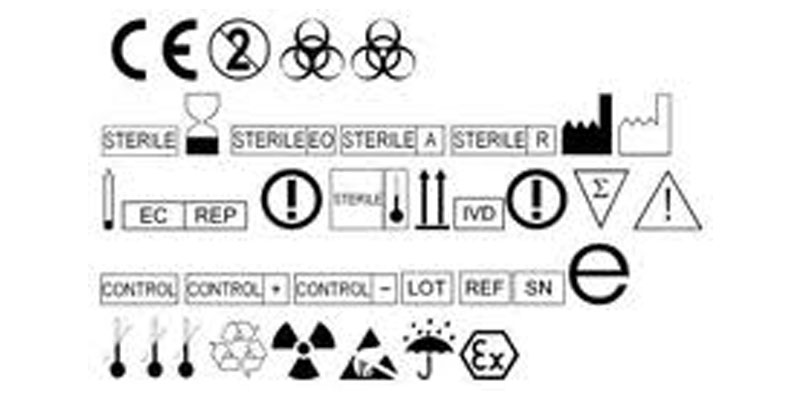
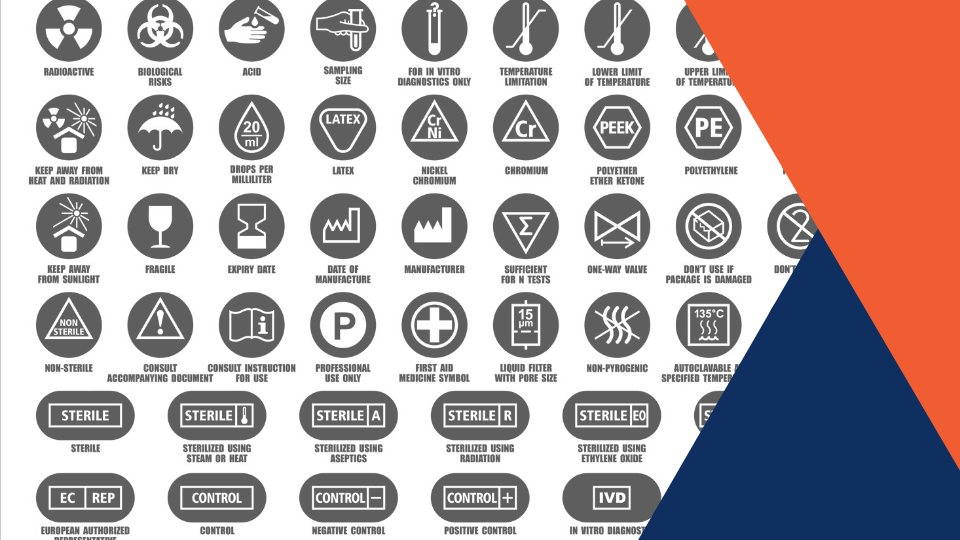
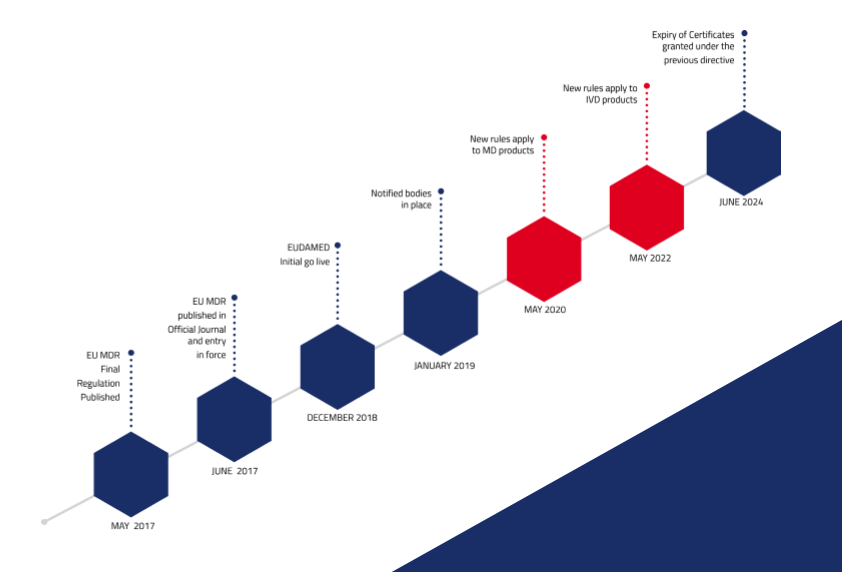
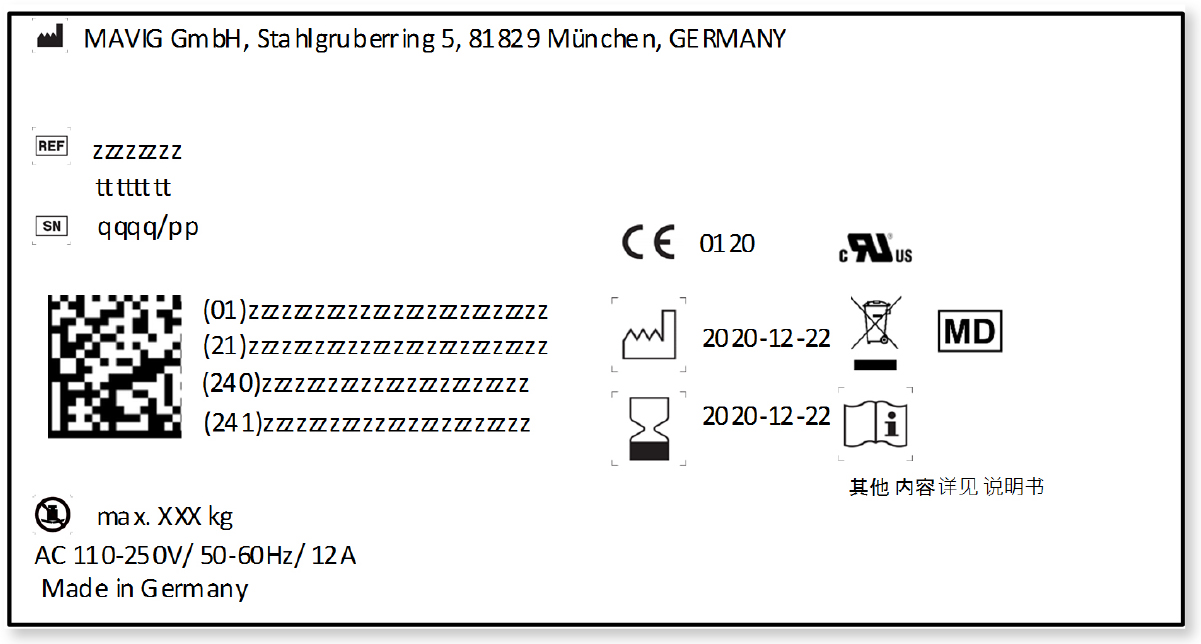
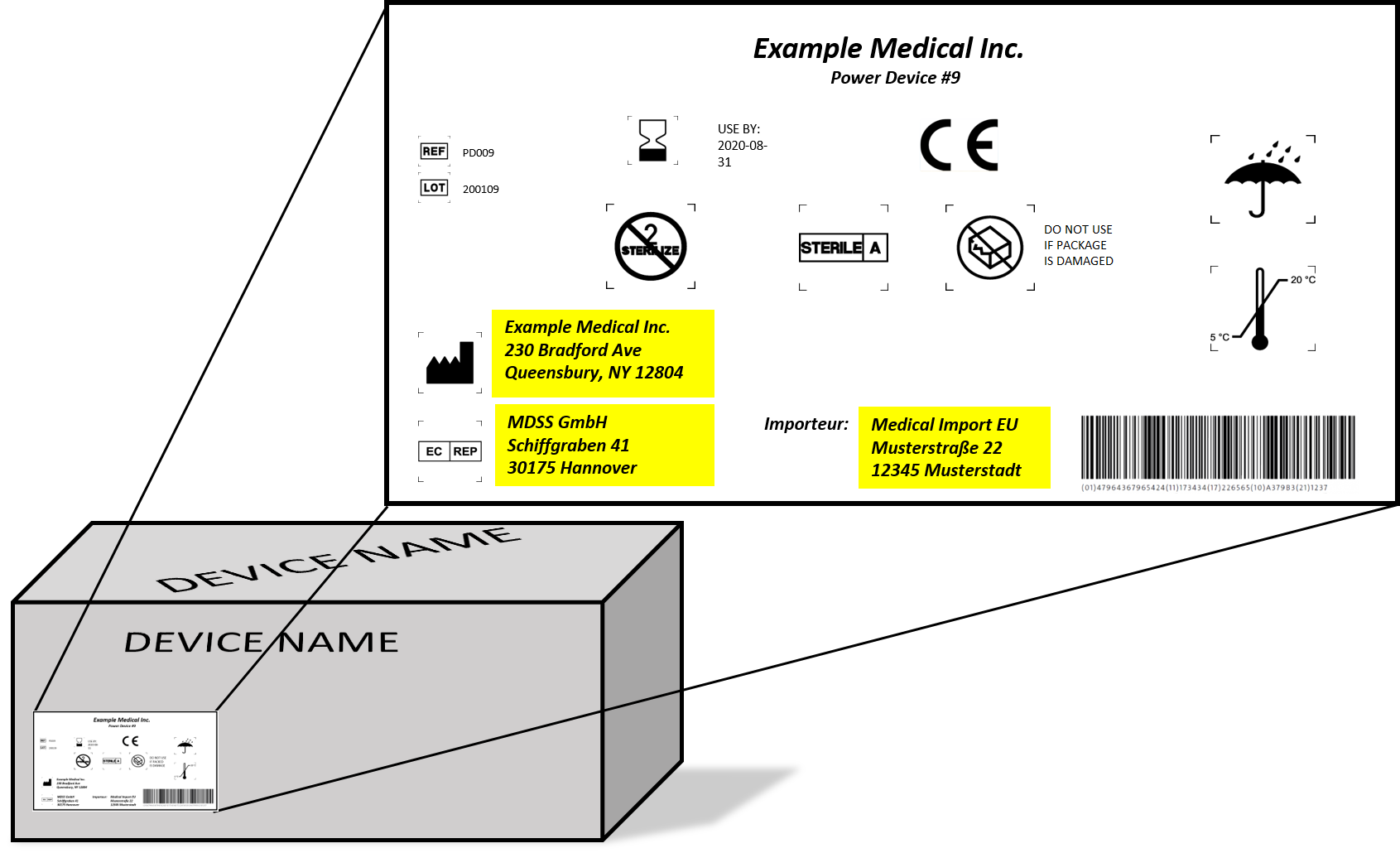
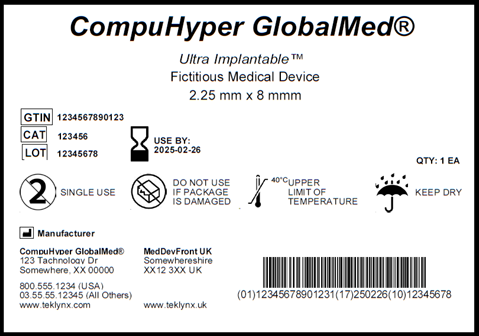
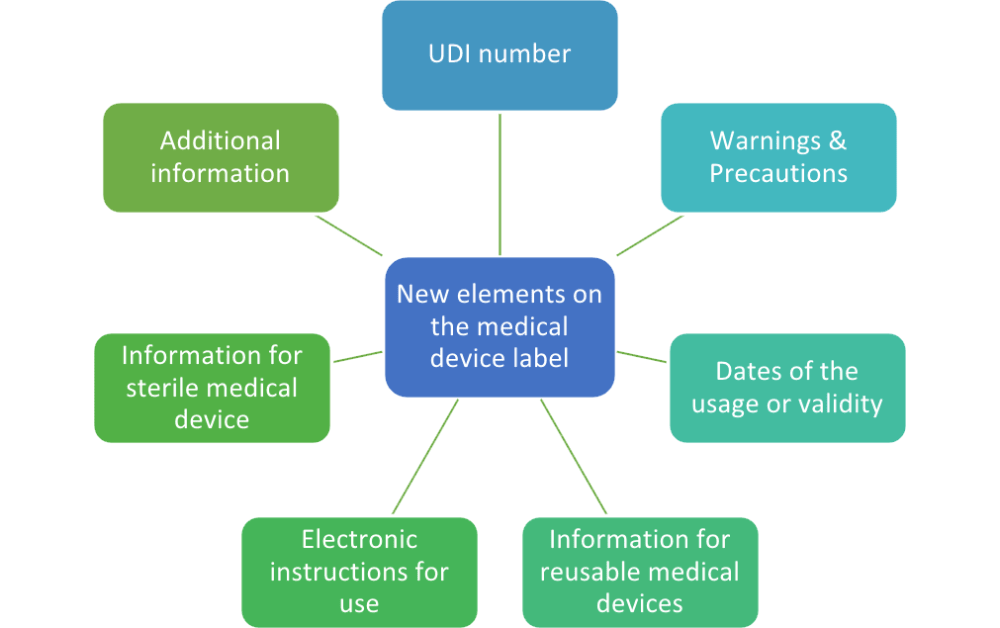
How can a manufacturer comply with such requirements within the short term and in a harmonized manner?

UDI & EUDAMED Explained under EU MDR - Clinical, Regulatory & Automation solutions for Life Sciences

MDR Checklist Labelling & IFU Requirements · MDlaw – Information platform on European medical device regulations